Editor’s note: This is part two in a two-part series.
An Anahola resident who underwent a clinical trial in 2007 for a then-experimental psoriasis drug is saying things went terribly wrong almost immediately after dropping out of the program early due to a heart complication.
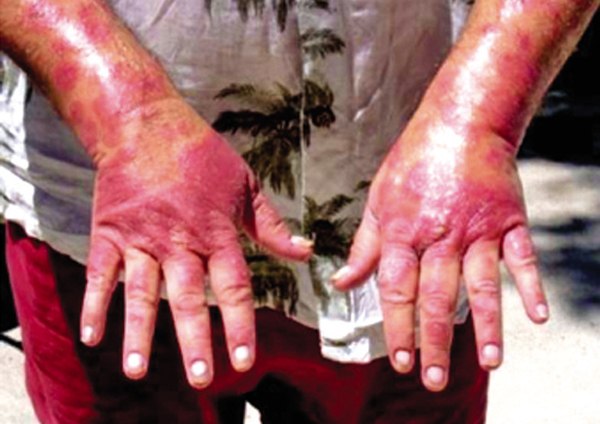
Tony Viscosi said more than 20 doctors told him it was Stelara — now federally approved — that caused his psoriasis to come back five-fold, nearly killing him.
ANAHOLA — Nearly six years after dropping out of the clinical trial, Viscosi’s psoriasis flares up regularly.
Walking or sitting is extremely difficult — and impossible at times — due to the sores on his feet and buttocks. He spends most of his time laying on his bed, in pain and bleeding.
His body has been back at 90 percent coverage on three flare-ups, he said. Usually he remains at 50 to 80 percent body-coverage. Lately, the psoriasis has been creeping inside his body.
In 2011, a grapefruit-sized growth developed on muscle tissue on Viscosi’s left leg.
That same year, an immune reaction required surgical removal of all but four of his teeth. Nerves grew throughout his mouth, causing severe pain, something his dentist had never seen in 30 years of practice.
Nerves and blood vessels grow into Viscosi’s nails, turning a simple task of clipping nails extremely painful.
Last year, a spot developed in his right lung.
Doctor after doctor denied treatment to Viscosi due to lack of information. He said each doctor told him Johnson & Johnson has the information and resources to investigate and treat his immune system breakdown.
Johnson & Johnson’s case manager who contacted Viscosi requested his medical records — the same records that Viscosi had sent the company prior to the clinical trial, he said.
Pat Kittler, a friend of Viscosi helping him through the ordeal, said it would cost thousands of dollars and countless hours to order Viscosi’s medical records. But Johnson & Johnson could have it for free, he said.
In the last few years, Viscosi and Kittler have spent at least $250,000 in medical expenses related to Stelara’s alleged adverse reactions. They were only able to pay for it because they liquidated all their assets, including homes, properties, a business and cars. They even cashed in their entire 401K retirement fund.
“I live on $700 a month that I get for supplemental Social Security,” Viscosi said. “I can’t even get my full Social Security because they don’t have a name for the disease that I have. I should be getting $1,800 a month, that’s what I paid into.”
Viscosi said he gets federal Medicaid, which pays for medical bills, but doesn’t approve everything. He said he has been waiting for two years for a wheelchair to help him get around.
Viscosi said Stelara costs at least $30,000 a year. The drug is projected to bring the company $1.47 billion in 2013, and gradually increase profits, reaching $2.74 billion by 2016.
The drug’s producer is Janssen Biotech.
Brian Kenney, director of public affairs at Janssen Global Services, said without having complete information of Viscosi’s situation and history, it would be inappropriate for the company to speculate who should pay for Viscosi’s medical bills. But Johnson & Johnson wants to reconnect with Viscosi to understand his health situation and assess how they may be able to help him, he said.
‘No big deal’
In 2008, when things first started to go wrong, Viscosi said he wasn’t scared.
“All I could think of was, ‘Oh, thank God, Johnson & Johnson is the biggest company in the world, as long as they’re with me I’m sure that this will be no big deal, they will know what’s wrong, they’ll fix it,’” he said.
After repeated attempts to seek help, he said he slowly found out the company didn’t seem to care.
“If you call, you better bring your lawyer,” he said.
But the company said that isn’t true.
“Patient safety is of the utmost importance to us and we take individual accounts of adverse events potentially related to our products very seriously,” Kenney said.
The company, Kenney said, strives to improve the quality of life of people living with plaque psoriasis and psoriatic arthritis, and to ensure the continued safe and effective use of Stelara as a treatment.
Viscosi also sent letters to Johnson & Johnson CEO Alex Gorsky, seeking medical help. He said the only response he got was from a company lawyer. But he is not interested in lawsuits, he just wants to receive treatment, which he says was in the clinical trial agreement he signed.
But Kenney said it wasn’t the company who stopped contact between the parties.
Kenney said a “case manager” was appointed in June 2012 — following Viscosi’s outreach to Gorsky — to work in collaboration with physicians to review all the available information.
“Unfortunately, we never received further information as requested in July 2012 of Mr. Viscosi through Mr. Kittler, which would have allowed us to more thoroughly review the situation and the reasons for his current health status,” said Kenney, adding he never heard from Kittler again until August 2013.
Kenney said Janssen remains interested in obtaining the information requested in 2012 to fully understand Viscosi’s current health situation and to assess how they may be able to assist him.
But Kittler disputes that.
He said he sent medical release forms to the pharmaceutical company, as requested, and informed them of the two hospitals which treated Viscosi. After two months of trying to get answers from Johnson & Johnson, Kittler said he gave up seeking their help.
So in August, Kittler put together a petition at www.change.org and sent copies to Gorsky, he said.
“We’re not trying to hide anything here, we need Tony to get treatment,” Kittler said.
Seeking help
Viscosi said he, his mother and Kittler filed at least 10 adverse reaction reports with the FDA in the last five years, but never heard back.
Stephanie Yao, of the FDA Office of Media Affairs, told The Garden Island when adverse event reports are submitted directly to the FDA via MedWatch, an acknowledgment of receipt is sent to the reporter.
“This acknowledgment does not include a response to specific questions regarding the adverse event,” she said. “When an adverse event report is submitted to the FDA by a manufacturer, the FDA does not acknowledge receipt of the report.”
Viscosi and Kittler said they also sought help from four U.S. senators, including Sen. Brian Schatz from Hawaii. Despite an interest from Schatz’s staff in January — immediately after Schatz was sworn in — they never followed up, and ignored 10 emails and follow up calls, Kittler said.
Julie McClain, Schatz’s press secretary said after Kittler contacted Schatz’s office, they filed an inquiry with the FDA to better understand Viscosi’s situation and what options or resources might be available to him.
“Our inquiry is still ongoing, and we hope that the FDA will provide additional information that will be helpful to the Viscosi family,” she said.
Without Viscosi’s violent reaction on the record with the FDA or Johnson & Johnson, the potential devastating side effects that Stelara could have on others have been muted, Viscosi said.
Kenney said any potential adverse reaction related to Stelara is documented by the company.
“We regularly communicate information and data from clinical trials and post-marketing registries to inform health care professionals and patients about the efficacy and safety of Stelara and the benefits and risks of treatment,” he said.
Yao said information from all sources contribute to the evaluation of all clinically relevant cases that can provide further understanding on safety and may also result in recommendation of regulatory actions. Those actions include a label update, a Risk Evaluation and Mitigation Strategy or restricting use pending further studies, or even market withdrawal.
“The FDA regularly publishes any emerging safety issues to inform the public and prevent or minimize the risk of harm,” Yao said.
But without help, Viscosi said his body will give out before his mind does. And that has pushed him to contact Oregon state to ask for death with dignity — assisted suicide — which is legal there.
Meanwhile, friends and families posted a petition at www.change.org asking President Barack Obama and the Congress for a “tiny speck” of Stelara’s profit to be put toward a solution for Viscosi.
Kittler said he may go to Washington D.C. later this year to protest before Congress.
Viscosi said whatever Johnson & Johnson can do to help him is going to help many others down the road. It’s not just him, he said.
“I don’t think this is isolated, as me being the one in a million,” he said.
Visit www.helpfortony.org to see a progression of Viscosi’s condition.
• Léo Azambuja, staff writer, can be reached at 245-0452 or lazambuja@thegardenisland.com