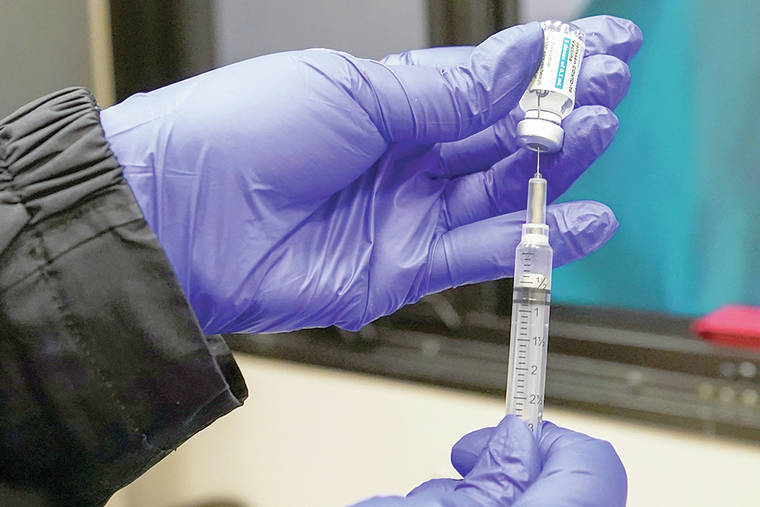
HONOLULU — Hawai‘i health officials are putting a pause on Johnson & Johnson COVID-19 vaccine distribution until further notice following an announcement from the Centers for Disease Control and Prevention suggesting a halt to all J&J vaccines.
But the COVID-19 vaccination rollout is continuing throughout the state.
The suspension comes after the CDC and the federal Food and Drug Administration said they were investigating unusual clots that occurred six to 13 days after vaccination with the J&J vaccine.
The rare clots occurred in six women out of the more than 7.2 million adults who’ve received the shot. One of the patients died and another remains hospitalized in serious condition.
The acting FDA commissioner said Tuesday she expected the pause to last a matter of days.
Most people vaccinated in Hawai‘i have received the Pfizer or Moderna vaccines, which each require two doses. The J&J vaccine only needs one dose.
Tuesday, state Department of Health Director Dr. Elizabeth Char said Hawai‘i was scheduled to receive only 2,600 doses of the J&J vaccine this week, so the pause won’t significantly disrupt the state’s vaccination plans.
Sites that were set to use the J&J vaccine will switch to the Moderna vaccine, for the most part, Char said, with some sites also using the Pfizer vaccine.
“(We) wanted to reassure our community that we’re vaccinating safely across Hawai‘i, and we want to encourage people to still get vaccinated,” Char said on Tuesday. “This is a setback for us. But we really, we still want people to get vaccinated to be protected for themselves and also protect their families from COVID.”
According to Char, the state received 47,000 J&J vaccines a month ago, and has administered over 17,000 vaccines statewide, leaving 30,000 single doses on hold.
“We’ll keep it in safe storage at the proper temperature control,” Char said, “and just wait for the science and the data and see where we go from there. Whatever the guidance is, if it’s suited better for certain populations or not at all.”
According to the Kaua‘i District Health Office, over 3,000 J&J doses were administered on Kaua‘i between Mar. 15 through April 12.
Char said Hawai‘i still expects to meet the Biden administration’s target of opening vaccinations to everyone 16 and older beginning Monday.
So far, 32% of Hawai‘i’s population has received at least one vaccine dose.
“My staff and I are closely monitoring reports that six recipients of the Johnson &Johnson vaccine in the United States have developed cases of a ‘rare and severe’ type of blood clot,” said U.S. Rep. Kaiali‘i Kahele said.
According to Kahele, as of April 12, more than 6.8 million doses of the J&J vaccine have been administered in the United States.
“Although six reported cases out of 6.8 million vaccine recipients represent an extremely small number, until we have more information I fully support the CDC and FDA’s decision to put the J&J vaccine on pause out of an abundance of caution,” said Kahele. “In Hawai‘i, the J&J vaccine has been widely used in recent weeks due to the convenience of a single-shot dose.”
Kahele urges those who received the J&J vaccine and develop a severe headache, abdominal pain, leg pain or shortness of breath within three weeks after vaccination to contact their health-care provider.
“Although these adverse events of blood clotting appear to be extremely rare, the safety of the COVID-19 vaccine is a top priority for the federal government,” Kahele said.
“Confidence in the vaccine is built on having a system that transparently addresses adverse events seriously, investigates them and enacts data-driven decisions, which is exactly what our federal agencies are doing right now.”
Char said she was unaware of anyone in Hawai‘i developing severe reactions to any of the COVID-19 vaccines.
“Six cases out of 6.8 million doses is very rare,” Char said.
•••
Stephanie Shinno, education, business and community reporter, can be reached at 245-0424 or sshinno@thegardenisland.com. The Associated Press contributed to this report.




If you happen to have adverse reactions to any of the vaccines approved by emergency use clause. Not an fda approved vaccine. Yet. You have no rights to sue or hold any of the companies liable for the adverse reactions. Now you may be able to sue business owners for making a shot mandatory in order to stay employed. Know your rights and do what’s best for yourself and family.
This is rare. But blood clotting are used in patients who’ve just come out of surgery. Such as any surgery requiring stitches. They use this to keep the blood from running out. The blood clots in such cases are part of the healing process. However, COVID-19 is different. No surgery is done. The patient is given a vaccine. Medicine for a virus. It probably was a surgical patient that received the vaccine. She died. I think it is rare cases like this.
Where’d you pull that opinion out of? Some serious mental gymnastics to connect all those hypothetical dots eh. Haha.
Vaccines all made for Covid at warp speed, no human testing, rush to judgement, experimental scary gene therapy getting people sick and injured, that’s right the pharmaceutical companies cannot be sued for vaccines injuring people, because they are protected by laws our elected officials created to protect corporations, instead of protecting the people who elected them.
Vaccines do not provide immunity to Covid, nor cause you to be non-contagious to Covid, nor allow you to develop Herd Immunity.
Only the natural disease virus can cause your body to become immune to Covid, and become non contagious, and Herd Immune, all within 2-3 weeks of infection with the natural Covid virus, and 98% of those infected have zero to mild symptoms.
The body’s immune system, when infected by any disease virus like Covid, has millions of Plasma cells that produce 10,000 Antibodies…per second, times millions of plasma cells over 2+ weeks period of time, that is over725 Quadrillion (725,000,000,000,000,000) Antibodies, and that is if there is only 1 million plasma cells.
When in fact there could be many or hundreds of millions of plasma cells producing 10,000 antibodies per second for 2 weeks, allowing us when we become become Covid positive, to wipeout the Covid disease virus in our bodies, and the remaining unused antibodies, still in our bodies and still “on guard” and are what protect us from a second same virus infection, meaning we become immune, that is protected from the same disease.
And because all the Covid virus are eliminated during the average 2 week Immune Process, there are no more virus to spread by that now immune person, so they are now Non-Contagious and safe to travel with no need of mask.
Being Immune and Non-Contagious makes a person have Herd Immunity, which eliminates a virus from the population.
Because antibodies bio-identically match the virus, like 2 same fingerprint matches, with the 725 Quadrillion antibodies eliminating all the Covid virus, the man made laboratory Vaccines do not match the Covid virus and do not elicit the Immune Response against the Covid virus, but instead the human body has to protect itself with it own antibodies against the vaccine itself.
Thus you get immunity against the vaccine but not against the Covid virus.
What a waste of time and dangerous use of a fake vaccine. It’s as insane s a 20 fake WAR we are again walking away from in Afghanistan like we did in Vietnam. What a waste in human life and Treasure.
And while the military industry made untold billions of $$$ dollars, off the Vietnam and Afghanistan Wars, so too is the pharmaceutical industry making billions off the hysterical fear of an annual virus and useless list of dangerous vaccines.
People are not dying from the virus, they are dying from their multiple food induced pre-existing end-of-life diseases and their prescription drugs, now complicated by the fake vaccine.
80% of those who died while positive with Covid were already over burdening their immune system with being obese along with the cousin diseases of diabetes, heart, stroke, and high blood pressure diseases, and their toxic and lethal prescription drugs they take dily for decades until death by disease and drugs.
Forget the vaccine, Get Healthy, see a Health Coach, Stay away from medications and prescription drugs, and dangerous fake vaccines that burden your immune system.
“Come on”, every doctor should know that even though only 6 people get sick or die out of 150 million people vaccinated, as time goes by more and more people over the next 15 years, more and will get sick and die.
Not even that 15 minutes making you wait after the shot for a vaccine bad reaction, we are talking about something that is long term bad reaction, development of cancer, blood clots on the brain, stroke, something bad that no one would associate with the vaccine today.
A typical 16 year old today that they are trying to rush in for a vaccine would only be 31 when a vaccine caused blood clot on the brain may cause stroke or any other severe disease or injury.
Now 15 years later, that 16 year then but now today is bed ridden with, with blood clots and, and you can’t sue the pharmaceutical company with the flu law protecting them.