• Editor’s note. This is part one in a two-part series
ANAHOLA — Born with good looks and an athletic disposition, Tony Viscosi had always been “part of the gang.”
Active with friends, the Southern Californian first moved to Kauai when he was 18 where he surfed and played sports.
The island was the perfect spot for the recreationalist.
But today he no longer surfs, he no longer play sports and has a diffucult time enjoying the island’s outdoor wonders.
It’s because, according to the 45-year-old Anahola resident, a clinical trial gone-wrong nearly six years ago changed everything. Now Viscosi is even considering assisted suicide.
He said a clinical trial in 2007 for a then-experimental psoriasis drug went terribly wrong almost immediately after he dropped out of the program early due to a heart complication.
“All it does, it takes you on a spiral downhill,” Viscosi said of Stelara, the drug since approved by the Federal Drug Administration. “It gives you about five good years until you’re finished. Unless they come up with something between now and then, you’re going to end up like me, on a bed waiting to die.”
On Sept. 25, 2009, the FDA approved ustekinumab, marketed as Stelara, to treat plaque psoriasis. Stelara is produced by Janssen Biotech (formerly Centocor Ortho Biotech), a subsidiary of Johnson & Johnson. To date, it has been approved in 74 countries to treat plaque psoriasis.
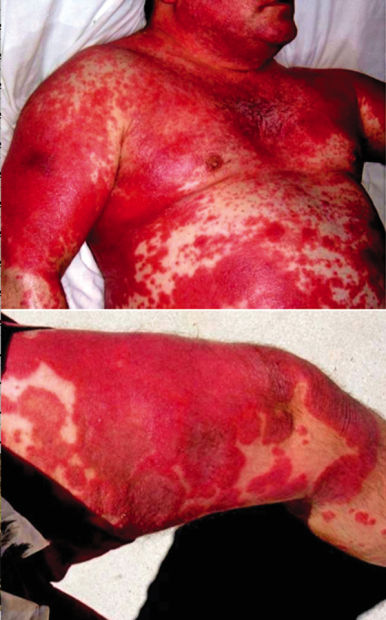
But a year before the FDA approved Stelara — and just less than one year after he took it — Viscosi was laying on a hospital bed, with 90 percent of his body covered in sores. Towels wet with sterile saline solution wrapped around Viscosi’s nearly skinless body kept him from dying. Even now, nearly six years after dropping out of the clinical trial, Viscosi’s psoriasis flares up regularly. Walking or sitting is extremely difficult — and impossible at times — due to the sores on his feet and buttocks. He spends most of his time laying on his bed, in pain and bleeding.
Psoriasis is a noncontagious auto-immune condition caused by molecular mimicry. Mild cases look like skin rashes, or peeling sunburns. But in Viscosi’s case, it has disfigured his body.
He said he has been kicked out of several stores, from a restaurant, and has been placed on an isolated seat on an airplane. In August, Pat Kittler, a friend of Viscosi helping him through the ordeal, and Viscosi paid to move into a Kalaheo home, but when the landlord saw Viscosi he called the deal off. Viscosi called it “a loss of dignity.”
“I had never in my life been discriminated against until I had this, and I was blown away,” Viscosi said. “All of a sudden, I was different.”
And he said he is sure that the drug is responsible.
He said more than 20 doctors told him it was Stelara that caused his psoriasis to come back five-fold, nearly killing him.
Yet, his plea for help has been ignored by just about everyone, from researchers to drugmakers to lawmakers, he said.
And without help, Viscosi said his body will give out before his mind does. It’s pushed him to contact Oregon state to ask about death with dignity — assisted suicide — which is legal there.
“I don’t want to be laying in some room just waiting (to die),” he said.
From diagnosis to experiment
At 32 years old, Viscosi was diagnosed with psoriatic arthritis, and two years later he developed plaque psoriasis.
He said he likely inherited plaque psoriasis from his father, who was diagnosed at 35 years old and has lived with it for 30 years. His father’s case stabilized at 10 to 15 percent body coverage, the same degree of Viscosi’s psoriasis prior to the clinical trial. But Viscosi’s father never developed psoriatic arthritis.
Though there is no cure for psoriasis, there are treatments available, including a controlled diet, nutritional supplements, oils and drugs.
In the first three years after being diagnosed, Viscosi took an immunosupressant and chemotherapy drugs, but they caused more side-effects than results, so he switched to less aggressive treatments. Viscosi said his psoriasis was mild, but the arthritis — and the drugs he took for it — caused him to go onto disability in 2001.
In early 2007, Viscosi entered into a five-year clinical trial of Stelara for plaque psoriasis, hoping it would also address the arthritis, since both conditions are linked by a high amount of a certain blood factor.
The trial was conducted in California by Therapeutics Clinical Research (TCR). In a matter of a few months, Stelara cleared Viscosi’s skin, but didn’t reduce his psoriatic arthritis.
Then in late 2007, Viscosi dropped out of the trial under his doctor’s recommendation, because an electrocardiogram detected something wrong with Viscosi’s heart.
In February 2008, tiny red spots, guttate psoriasis, appeared all over Viscosi’s body. By March 2008, the spots had grown much larger.
By May 1, 2008, Viscosi said he had called TCR several times and had sent letters with pictures showing his condition, but received no replies.
On May 15, 2008, he was admitted to a hospital for the first time. He had developed infections all over his body, caused by cracked skin, which bled constantly.
By August 2008, Viscosi was clinging to life, with about 90 percent of his body covered in sores and with nearly no skin left. He had to lay in bed for 20 hours a day, covered in towels wet with sterile saline to keep his fever down and avoid shock and kidney failure due to dehydration.
Around that time, three TCR doctors — research coordinator Barbara Doyle, staff physician Sandra Adsit and the company’s owner — examined Viscosi and told him his condition had nothing to do with Stelara, though they never took skin tissue or blood samples, he said.
The Garden Island contacted TCR over the phone and sent them questions via email, which were never answered despite several requests.
Stelara FDA approved
Since its first approval, Stelara went through several label revisions by the FDA.
Recently, it was approved to treat an additional condition, arthritis.
On Sept. 20 the FDA approved the drug to treat psoriatic arthritis, a joint inflammation that attacks about 30 percent of people with psoriasis, and the main reason Viscosi entered the clinical trial.
The drug’s producer is Janssen Biotech.
Janssen Medical Affairs Vice President Cindy Guzzo, in press release Sept. 23, praised the safety and efficacy of Stelara, calling it “a meaningful new option” for psoriatic arthritis patients.
Brian Kenney, director of public affairs at Janssen Global Services, told The Garden Island that Stelara demonstrated efficacy and safety in one of the largest clinical development programs ever conducted for a “biologic therapy” in the treatment of moderate to severe plaque psoriasis.
A biological therapy is a treatment with substances made from living organisms, according to the National Cancer Institute. Some biological therapies stimulate or suppress the immune system to help the body fight cancer, infection and other diseases.
Kenney said it would be “inappropriate” for the company to comment on Viscosi’s medical condition, but said that the drug has a track record of testing success.
“We have accumulated five years of efficacy and safety data,” Kenney said. “Stelara continues to demonstrate a positive safety profile, as demonstrated in the clinical trials.”
But to Viscosi, the drug is neither safe nor efficient.
The clinical trial Viscosi entered was to treat plaque psoriasis, which used to cover 10 to 15 percent of his body. He’d hoped Stelara would target psoriatic arthritis, a condition which made him go on disability.
He said Stelara had no effect on his arthritis, and once he dropped the trial, five other types of psoriasis developed all over his body.
Part two tomorrow: Seeking help
• Léo Azambuja, staff writer, can be reached at 245-0452 or lazambuja@thegardenisland.com